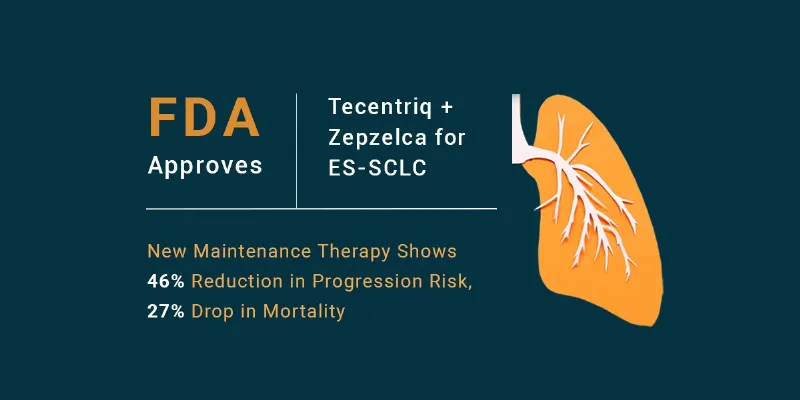
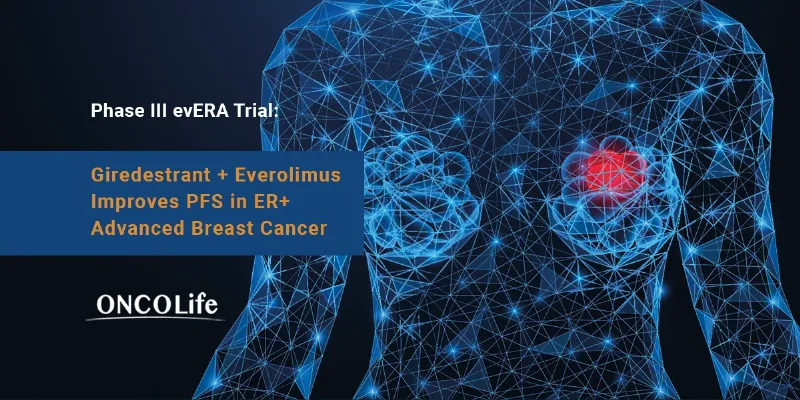
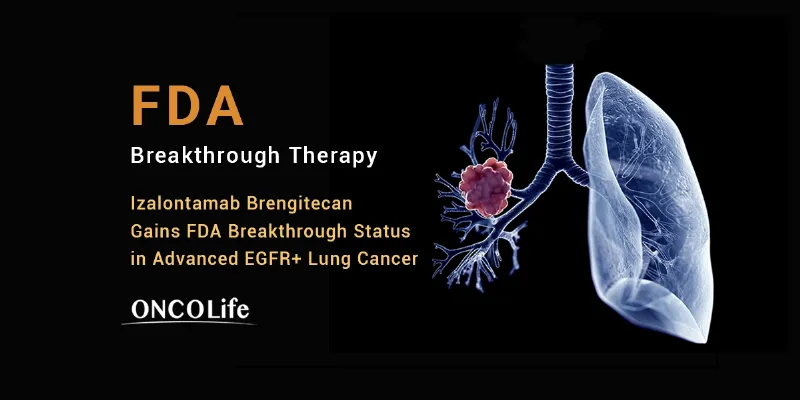
Health and Pharma
Editor
Health and Pharma is a new-generation international news and communication platform. We strive to be a top-tier trusted news outlet, delivering firsthand reports from reliable channels. Our goal is to collaborate with health science and pharma professionals.